MCC NEET UG Counselling 2024 dates for 15% AIQ seats today; NTA to revise merit

As per the Supreme Court ruling, the National Testing Agency (NTA) will update the NEET UG merit list 2024 by accepting the answer provided by a panel of three experts from IIT Delhi to a disputed physics question as the accurate one. Initially, when announcing the NEET results, the NTA awarded 4 marks for this question by considering two options as correct. However, now only those who selected the answer matching the IIT-D panel’s suggestion will receive marks. The revised NEET UG merit list 2024 will be available on the official website, exams.nta.ac.in/NEET/.
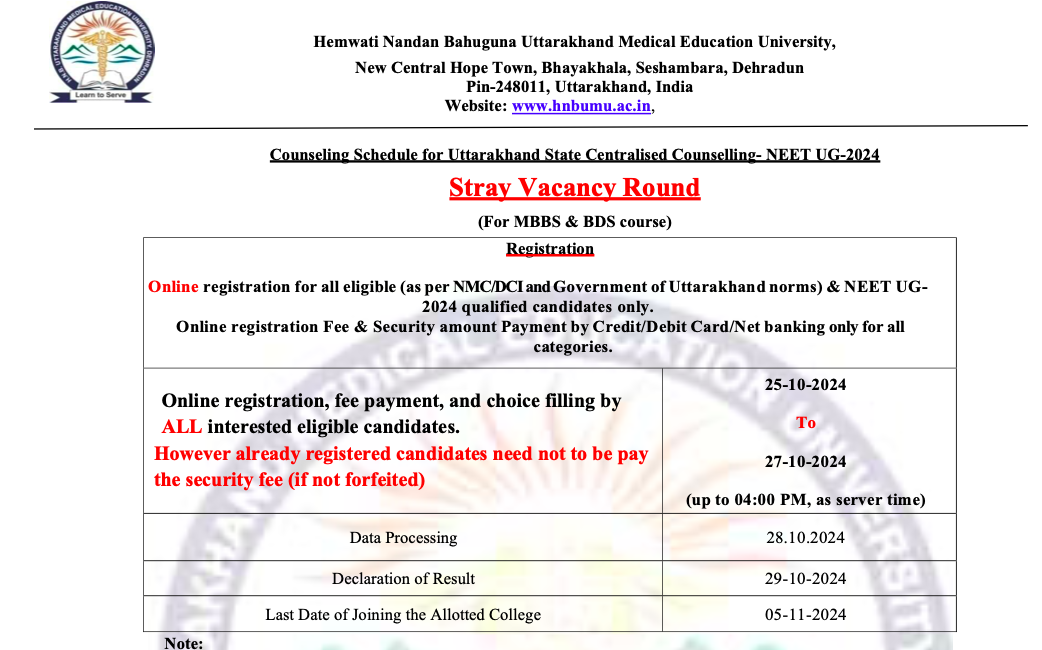
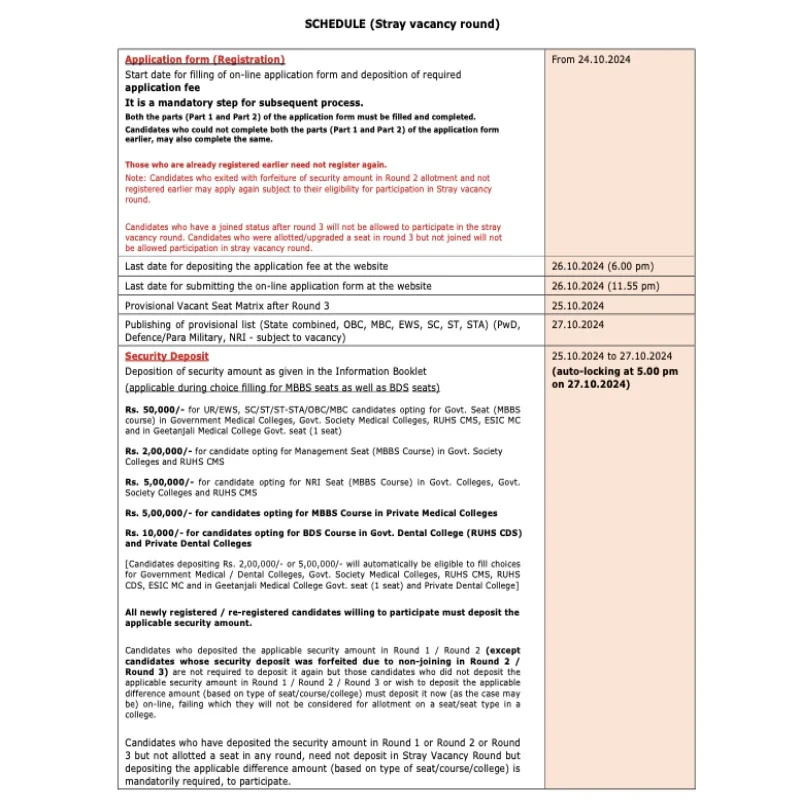
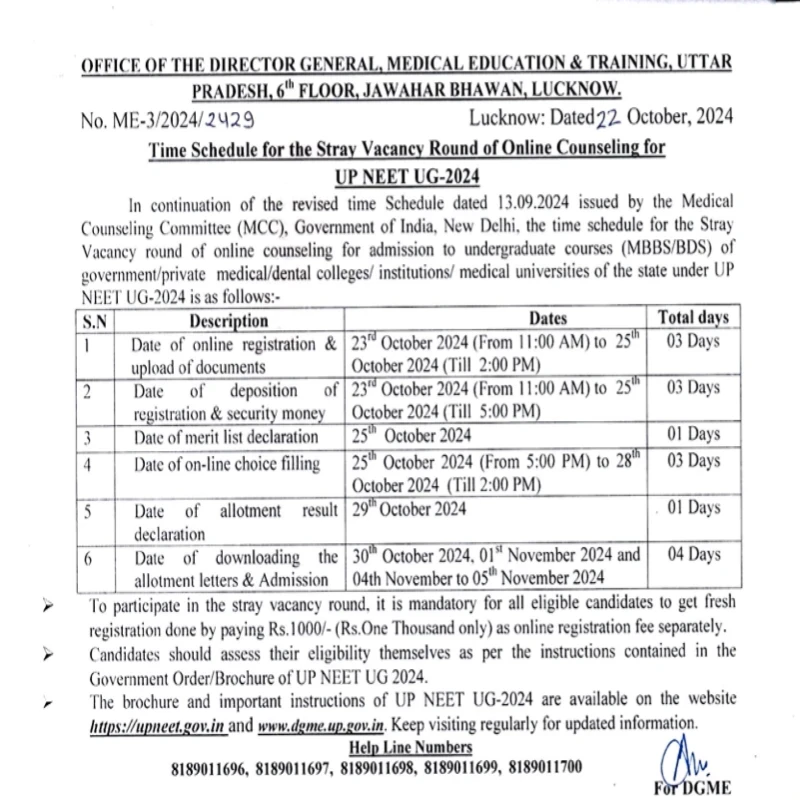
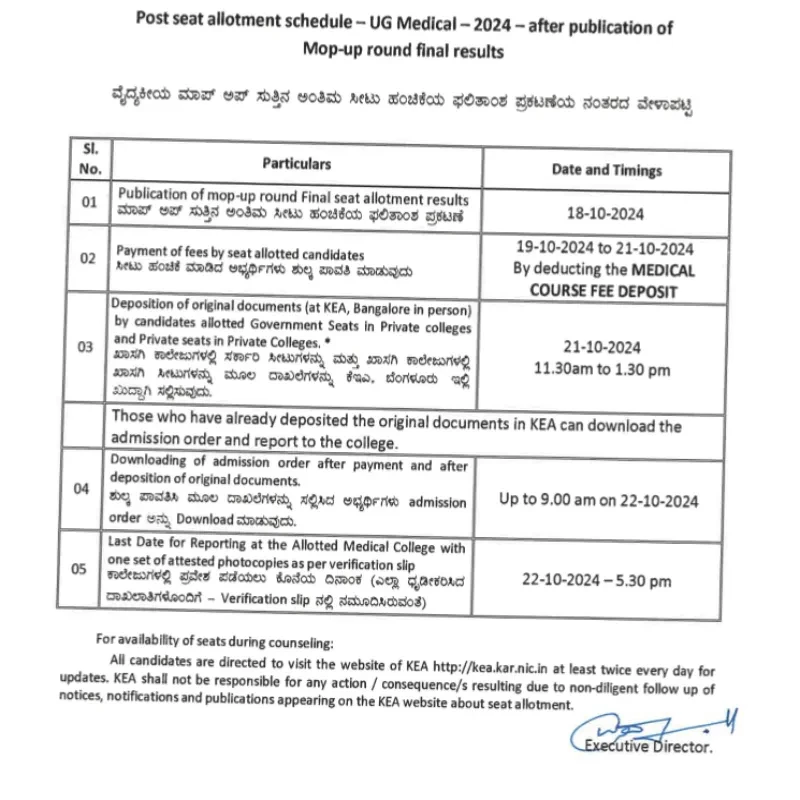
There will be four rounds of All India Quota (AIQ) online counselling. The allotments will be made by the MCC as per their merit and choice, which starts “only after receiving the list, data, or information of successful candidates from National Testing Agency”. The NTA is expected to issue the revised merit list soon.
The All India Quota (AIQ) online counselling will occur in four rounds. The Medical Counselling Committee (MCC) will make allotments based on merit and preferences, which will commence only after receiving the list, data, or information of successful candidates from the National Testing Agency (NTA). The NTA is expected to release the updated merit list soon.
The MCC will oversee the NEET UG counselling for the following institutions:
- 15% AIQ Seats: MBBS and BDS seats in various states.
- 100% Seats: MBBS and BDS seats at BHU.
- AIIMS: 100% MBBS seats at all AIIMS institutions across India.
- JIPMER: Open seats in Puducherry/Karaikal.
- AMU: Open seats.
- 15% AIQ Seats: Delhi University (DU), IP University (including VMMC, ABVIMS, ESIC Dental).
- Jamia Millia Islamia: Open seats at the Faculty of Dentistry.
- 15% AIQ Seats: ESIC medical colleges.
NEET Physics Question:
Consider the following statements:
- Atoms are electrically neutral because they contain an equal number of positive and negative charges.
- Atoms of each element are stable and emit their characteristic spectrum.
Based on these statements, choose the correct option:
- Statement I is incorrect, but Statement II is correct.
- Both Statement I and Statement II are correct.
- Both Statement I and Statement II are incorrect.
- Statement I is correct, but Statement II is incorrect.
Recent News
Become a member and stay up to date with our favorite topics and publications.